What is Titration , simple definition:-
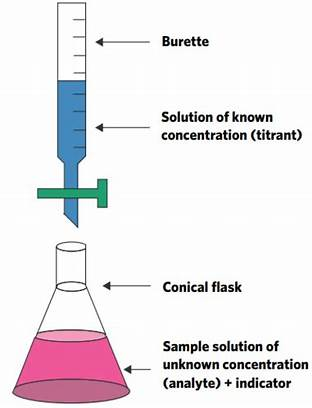
A titration is one of the most common quantitative lab techniques for determining the concentration of an unknown chemical. The power of the process is its simplicity, as there is a visual indication, a color change, that allows the reaction process to be monitored and the amounts of reactants measured. Pls. refer to typical lab set up for manual titration and graph on various types of acid base titrations.
HCl(aq.)+NaOH(aq.)→NaCl(aq.)+H2O(l)
With the innovation of technology over few decades, there are several manufacturing companies across the globe, who are providing titrators for automation, ease of calculation, high through put with use of sample changers, human errors reduced significantly with soft ware for GLP and these sophisticated titrators do comply with other critical compliances especially for pharma sector.
What are different types of Titration techniques?
Redox titration, on the other hand, involves an oxidation-reduction reaction
Complexometric titration is used to determine the concentration of metal ions in a solution.
Zeta potential titration is used to measure the surface charge of colloids
Isothermal titration calorimetry measures the heat released or absorbed during a chemical reaction.
Spectroscopy involves measuring the absorption or emission of light by a sample 1. Amperometry measures the current produced by a chemical reaction.
Gas phase titration is used to determine the concentration of a gas in a mixture.
Thermometric titrimetry measures the temperature change during a chemical reaction
Tips to improve Accuracy of Titration in your laboratory?
a) Standardize your Titrant: This is a procedure which normalizes the titration system and due to this exercise one gets accurate and reproducible results. Titrants example like Sodium Hydroxide tends to absorb gases in atmosphere and they change their strength hence it is very important that before start of titration, one must use either fresh titrant or standardize it with at least triplicate operations. A % RSD(Relative standard deviation) is calculated and a value of less than 1% is acceptable in most of GLP(gool laboratory practice) labs.
b) Homogenize sample: Depending upon sample under test should be homogenized example cheese, pharma tables etc. The homogenized samples make molecule accessible to titrant and hence accuracy of sample is quite certain.
c) Sample measuring Technique: If we go back to our school time we used volumetric flasks, volumetric graduated pipettes or weighing machines(balances). These were very important and still basics one should follow in lab whether he/she is going with manual or auto titrator. Why? pls see equation below.
Cs= Ct*Vt Ct: Conc. of titrant
Vs Vt: Volume of titrant used
Vs : Volume of sample (any error in taking sample will impact on accuracy)
For solid samples it should be weighed with calibrated balance, depends on the number of significant figures desired in the final results.
Calibrate Electrode/Sensor: It is obvious for a chemist however it is necessary to highlight if you are using an electrode
-pH for acid base
-Redox for ORP,
-Indicator electrode for EDTA/complexometric titration etc.
over a period of time and continuous usage of these electrodes, the response time increases which is an indication that they are due to calibrate and hence we suggest at least once a day the electrodes are calibrated before start of titration. This way you are sure that electrode is still with same accuracy and more so the electrode gets regenerated as well. It enhances it life.
Check for Air bubble in burette / dispenser : whether you are using manual or auto titrator technique, air bubbles can form in burettes. This can lead to false, low results since air is taking the space in the burette and measured volume of titrant is more than what actually consumed. In manual stopcock burettes, bubles are formed at its tip which can be removed by flicking it or purging several mls of titrant through it. In case of auto titrator one can purge/flush through either through preprogrammed or through manual push button on it.
*******************************************



Comments